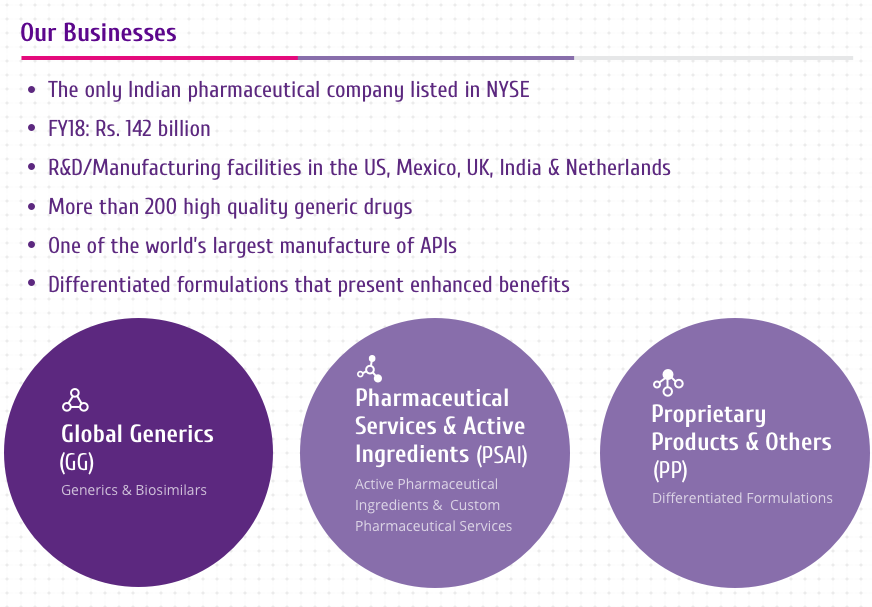
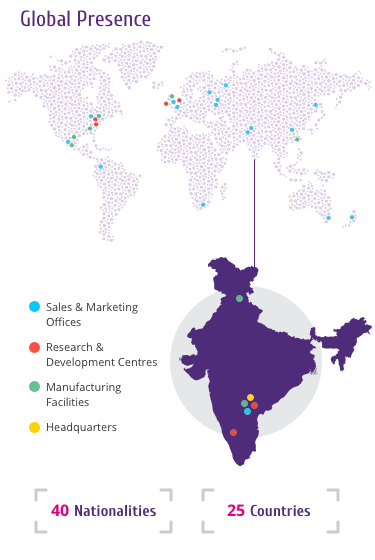
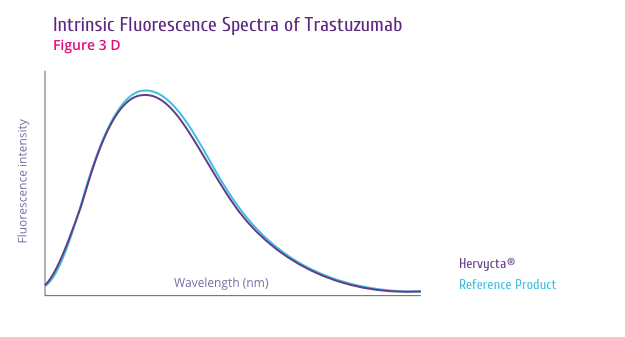
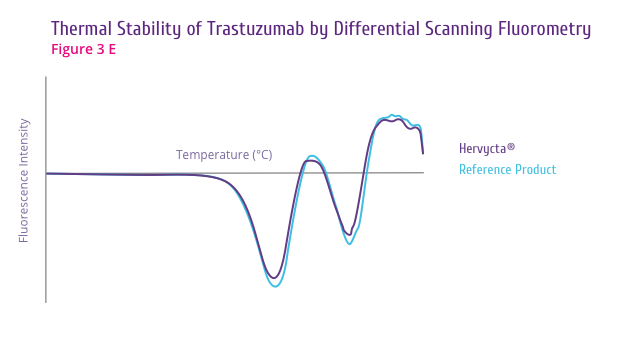
Trastuzumab
Trastuzumab is a HER2 targeting drug and is the active ingredient
of HERVYCTA®.
It is currently one of the few approved and effective treatment
options available for HER2+ breast cancers. Addition of
trastuzumab to the therapeutic protocol has strong benefits.
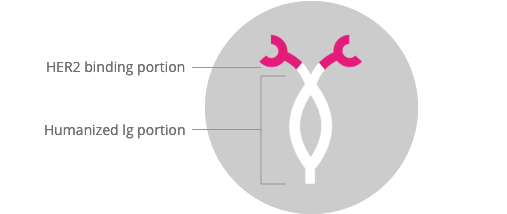
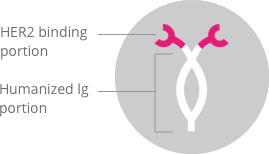
It is a humanized IgG1 monoclonal antibody which has been designed
to bind to the HER2 receptor protein on the cell surface[1-4]
(Figure 1).
Although effective, Trastuzumab may not work in some
individuals. There can be serious side-effects. It is important
that the patient is made aware of all possible side-effects.
Read important safety information
here.
HER2 gene and cancer
HER2, or, Human epidermal growth factor receptor 2 is a
transmembrane tyrosine kinase receptor that normally regulates
cell growth and survival. This protein is encoded by the HER2/neu
proto-oncogene. The HER2 gene can get mutated in certain cases
(amplified or over-expressed), resulting in cancer. This results
in an increased expression of HER2 receptors on the cell surface
and a consequent increase in cell signaling and proliferation
(Figure 2).
HER2 overexpression is observed in 15-20% of all human primary
breast cancers and in some cancers of the gastric and
gastroesophageal junction origin.
Figure 2:
HER2 receptor density on normal cells vs. HER2 positive cancer
cells
As the HER2 protein is amplified in these cancers (an alteration
commonly referred to as the HER2+ status), the normal signaling
outcome is also amplified in them. Thus, the HER2+ status confers
an enhanced survival advantage and metastasis-promoting
characteristic to the tumor. It is thus the reason behind the
aggressive biological behavior of these cancers. For this reason,
it is strongly associated with a poor prognosis[2-3].
Interestingly, HER2+ cancers have a therapeutic advantage,
attributable to the overexpressed HER2 receptor protein itself.
And this knowledge has paved way to the development of a class of
highly effective targeted therapeutics known as the HER2 targeting
drugs.
HER2 targeted therapy with Trastuzumab
The fact that normal cells and cancer cells express varying levels
of HER2, and that HER2 is attributed to disease progression, makes
HER2+ cancer an ideal target for targeted therapy. HER2 targeted
therapeutics preferentially target and bind to HER2 overexpressing
cancer cells. After preferential binding, these cells are then
directed to destruction.
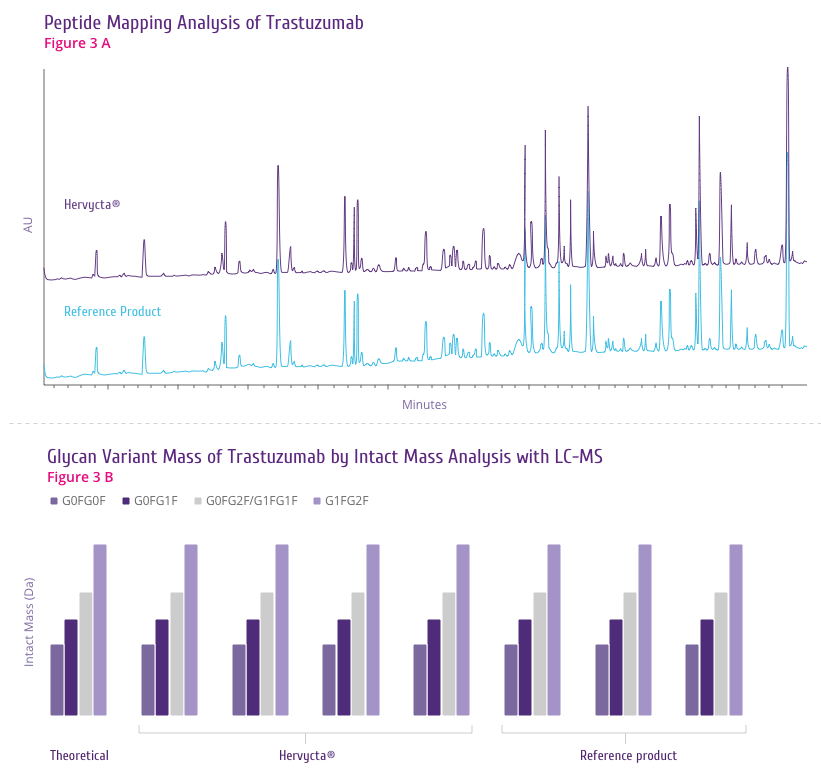
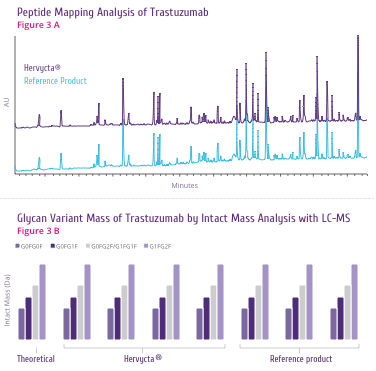
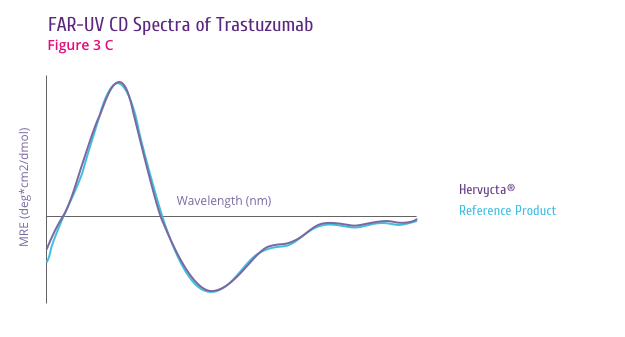
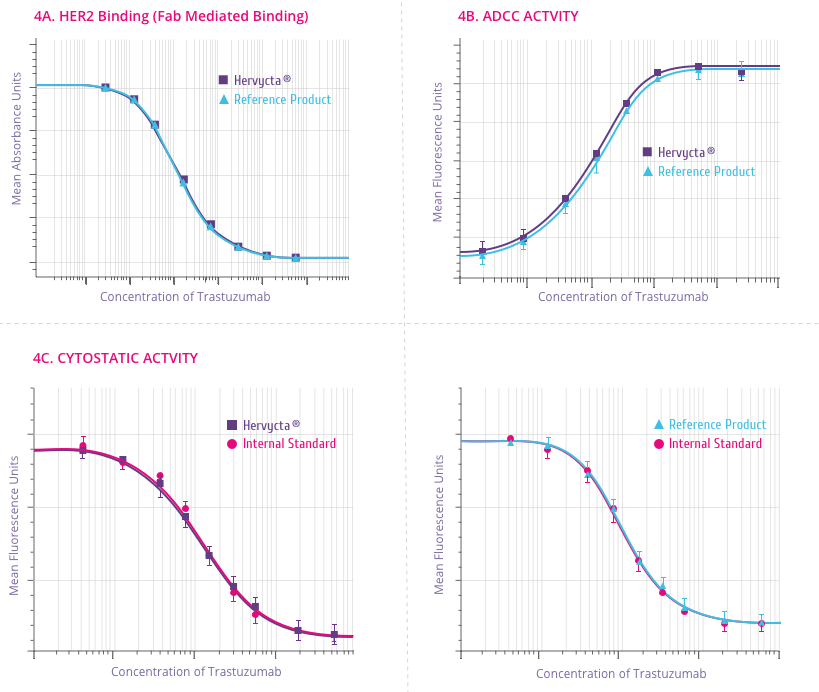
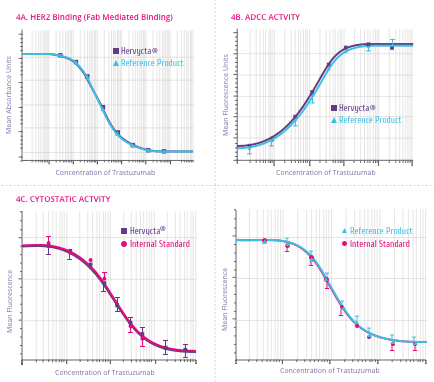
Trastuzumab is a highly effective HER2-targeting drug
(Figure 3).
Figure 3:
Trastuzumab binding shuts down HER2 signaling
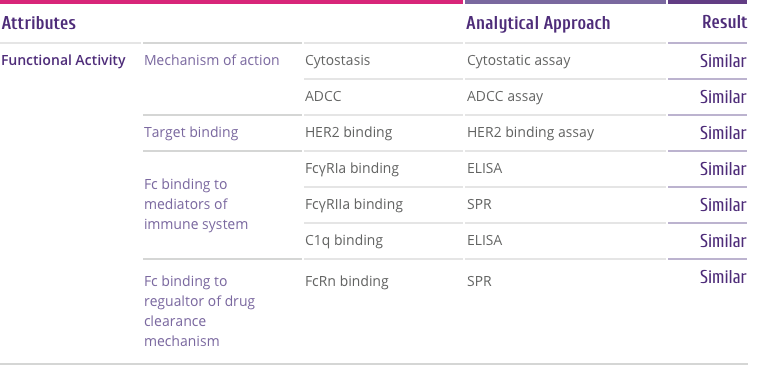
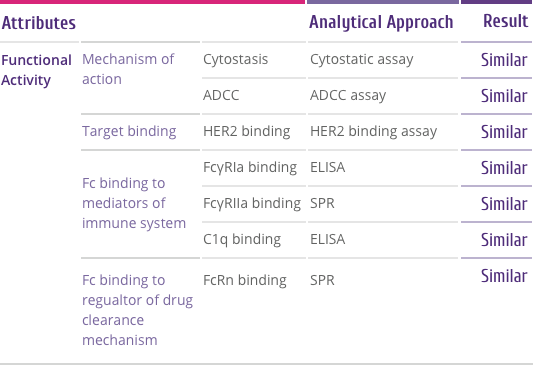
Mechanism of Action
How Trastuzumab works
Trastuzumab binds to the HER2 receptor at the extracellular
sub-domain IV, a juxta-membrane region of the receptor. This
binding inhibits HER2 signaling and also prevents proteolytic
cleavage of the extracellular domain (Figure 4). Below are
the outcomes of trastuzumab binding[5] .
-
Downregulation of HER2 activity
HER2 signaling normally culminates in increased cell
proliferation. Upon trastuzumab binding, this signaling
cascade is shut down. Also trastuzumab binding leads to
internalization of HER2 receptor from the cell surface in to
the cell through endocytosis.
-
Tumor cell lysis by Antibody-dependent cell-mediated
cytotoxicity (ADCC)
ADCC is believed to be one of the most potent mechanism of
action of trastuzumab. After binding to the HER2 receptor,
trastuzumab attracts Natural Killer (NK) cells to the site
via its Fc region, resulting in tumor cell killing by ADCC.
-
Suppression of Angiogenesis
Trastuzumab alters blood vessel development by inducing
regression.
-
Cell cycle arrest
Binding of trastuzumab leads to induction of cell cycle
phase related proteins, which creates a “cytostatic”
situation.
Figure 4:
Outcomes of Trastuzumab binding to the HER2 receptor[5]
Source:
Hudis CA, Trastuzumab – mechanism of action and use in
clinical practice, N Engl J Med., 2007, Jul 5:357(1):39-51
HER2: Human Epidermal Growth Factor 2
Indications
HERVYCTA® is indicated for the treatment of[6]
I.Early Breast Cancer (EBC)
HERVYCTA® is indicated for the treatment of adult
patients with HER2 positive early breast cancer (EBC):
-
Following surgery, chemotherapy (neoadjuvant or adjuvant) and
radiotherapy (if applicable).
-
Following adjuvant chemotherapy with doxorubicin and
cyclophosphamide, in combination with paclitaxel or docetaxel.
-
In combination with adjuvant chemotherapy consisting of
docetaxel and carboplatin.
-
In combination with neoadjuvant chemotherapy followed by
adjuvant trastuzumab therapy, for locally advanced (including
inflammatory) disease or tumours > 2 cm in diameter.
II.Metastatic Breast Cancer (MBC)
HERVYCTA® is indicated for treatment of adult
patients with HER2 positive metastatic breast cancer
-
As monotherapy for the treatment of those patients who have
received at least two chemotherapy regimens for their metastatic
disease. Prior chemotherapy must have included at least an
anthracycline and a taxane unless patients are unsuitable for
these treatments. Hormone receptor positive patients must also
have failed hormonal therapy, unless patients are unsuitable for
these treatments.
-
In combination with paclitaxel for the treatment of those
patients who have not received chemotherapy for their metastatic
disease and for whom an anthracycline is not suitable.
-
In combination with docetaxel for the treatment of those
patients who have not received chemotherapy for their metastatic
disease.
-
In combination with an aromatase inhibitor for the treatment of
postmenopausal patients with hormone‐receptor positive MBC, not
previously treated with trastuzumab.
III.Metastatic Gastric Cancer (MGC)
-
HERVYCTA® in combination with capecitabine or
5-fluorouracil and cisplatin is indicated for the treatment of
adult patients with HER2 positive metastatic adenocarcinoma of
the stomach or gastroesophageal junction who have not received
prior anti-cancer treatment for their metastatic disease.
-
HERVYCTA® should only be used in patients with
metastatic gastric cancer (MGC) whose tumours have HER2
overexpression as defined by IHC2+ and a confirmatory SISH or
FISH result, or by an IHC 3+ result.
Dosing & Administration
Dosing
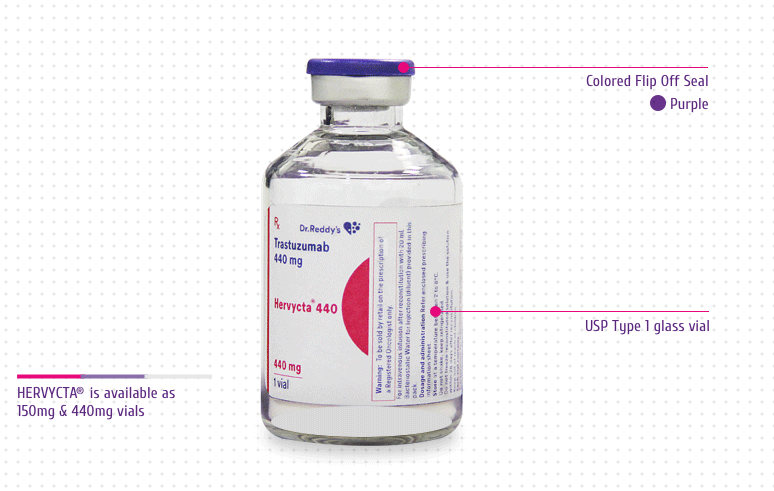
HERVYCTA® (Trastuzumab) is available as a
lyophilized powder for concentrate for solution for Intravenous
Infusion[6].
The active ingredient in HERVYCTA® is Trastuzumab
(recombinant DNA origin). HERVYCTA® is presented
in two dosage forms – 150 mg and 440 mg multiple dose vials, as a
sterile, white to pale yellow, preservative-free lyophilised
powder. Both the dosage forms are packed as follows:
-
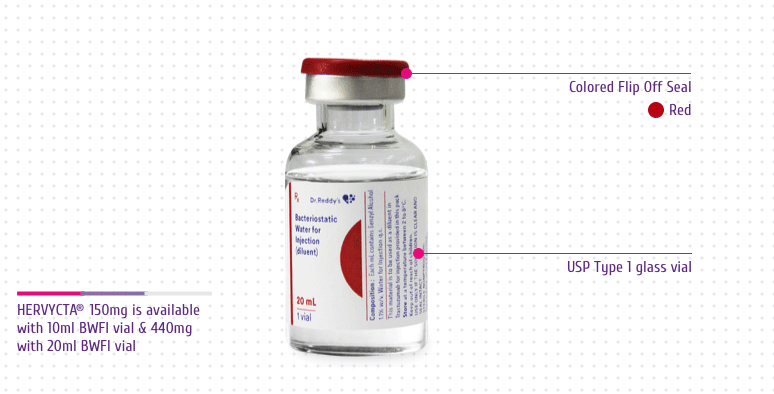
Combi pack of 1 vial of 150 mg Trastuzumab for Injection + 1
vial of 10 mL Bacteriostatic Water for Injection USP (diluent).
Multi-use vial for I.V. infusion Lyophilised powder
-
Combi pack of 1 vial of 440mg Trastuzumab for Injection + 1 vial
of 20mL Bacteriostatic Water for Injection USP (diluent), each.
Multiple dose vial for I.V. infusion Lyophilised powder.
HER2 testing is mandatory prior to initiation of therapy
(Detection of HER2 protein overexpression is necessary for
selection of patients appropriate for HERVYCTA® therapy
because these are the only patients studied and for whom benefit
has been shown in clinical trials).
Early breast cancer (EBC)
Weekly schedule and three‐weekly
As a weekly regimen (initial loading dose of 4 mg/kg followed by
2 mg/kg every week) concomitantly with paclitaxel following
chemotherapy with doxorubicin and cyclophosphamide.
As a three‐weekly regimen the recommended initial loading dose
of HERVYCTA® is 8 mg/kg body weight. The
recommended maintenance dose of HERVYCTA® at
three‐weekly intervals is 6 mg/kg body weight, beginning three
weeks after the loading dose.
Metastatic breast cancer (MBC)
Weekly schedule
The recommended initial loading dose of HERVYCTA®
is 4 mg/kg body weight. The recommended weekly maintenance dose
of HERVYCTA® is 2 mg/kg body weight, beginning
one week after the loading dose.
Three‐weekly schedule
The recommended initial loading dose is 8 mg/kg body weight. The
recommended maintenance dose at three‐weekly intervals is 6
mg/kg body weight, beginning three weeks after the loading dose.
Metastatic gastric cancer (MGC)
Three weekly schedule
The recommended initial loading dose is 8 mg/kg body weight. The
recommended maintenance dose at three-weekly intervals is 6
mg/kg body weight, beginning three weeks after the loading dose.
Duration of treatment
Patients with MBC or MGC should be treated with HERVYCTA®
until progression of disease. Patients with EBC should be
treated with HERVYCTA® for 1 year or until
disease recurrence, whichever occurs first; extending treatment
in EBC beyond one year is not recommended.
Dosage adjustments during treatment
No reductions in the dose of HERVYCTA® were made
during clinical trials. Patients may continue therapy during
periods of reversible, chemotherapy‐induced myelosuppression but
they should be monitored carefully for complications of
neutropenia during this time.
If left ventricular ejection fraction (LVEF) percentage drops ≥
10 points from baseline AND to below 50 %, treatment should be
suspended and a repeat LVEF assessment performed within
approximately 3 weeks. If LVEF has not improved, or has declined
further, or if symptomatic congestive heart failure (CHF) has
developed, discontinuation of HERVYCTA® should
be strongly considered, unless the benefits for the individual
patient are deemed to outweigh the risks. All such patients
should be referred for assessment by a cardiologist and followed
up.
Missed doses
If the patient has missed a dose of HERVYCTA® by
one week or less, then the usual maintenance dose (weekly
regimen: 2 mg/kg; three‐weekly regimen: 6 mg/kg) should be
administered as soon as possible. Do not wait until the next
planned cycle. Subsequent maintenance doses should be
administered 7 days or 21 days later according to the weekly or
three‐weekly schedules, respectively.
If the patient has missed a dose of HERVYCTA® by
more than one week, a re‐loading dose of HERVYCTA®
should be administered over approximately 90 minutes (weekly
regimen: 4 mg/kg; three‐weekly regimen: 8 mg/kg) as soon as
possible. Subsequent HERVYCTA® maintenance doses
(weekly regimen: 2 mg/kg; three‐weekly regimen 6 mg/kg
respectively) should be administered 7 days or 21 days later
according to the weekly or three‐weekly schedules respectively.
Method of Administration
HERVYCTA® loading dose should be administered as a
90-minute intravenous infusion. Do not administer as an
intravenous push or bolus.
HERVYCTA® intravenous infusion should be
administered by a health-care provider prepared to manage
anaphylaxis and an emergency kit should be available. Patients
should be observed for at least six hours after the start of the
first infusion and for two hours after the start of the subsequent
infusions for symptoms like fever and chills or other
infusion-related symptoms. Interruption or slowing the rate of the
infusion may help control such symptoms. The infusion may be
resumed when symptoms abate.
If the initial loading dose was well tolerated, the subsequent
doses can be administered as a 30-minute infusion.
Special precautions for disposal
Appropriate aseptic technique should be used.
Multiple dose vial (Multi-use vial) 440 mg
Each 440 mg vial of trastuzumab is reconstituted with 20 mL of
Bacteriostatic Water for Injection (BWFI), USP, containing 1.1%
benzyl alcohol as a preservative to yield a multi-dose solution
containing 21 mg/mL trastuzumab. In patients with known
hypersensitivity to benzyl alcohol, reconstitute with 20 mL of
Sterile Water for Injection (SWFI) without preservative, to
yield a single use solution.
Store reconstituted trastuzumab at 2°C – 8°C; discard unused
trastuzumab after 28 days. If trastuzumab is reconstituted with
SWFI without preservative, use immediately and discard any
unused portion
Instructions for reconstitution:
-
Using a sterile syringe, slowly inject 20 mL of Bacteriostatic
Water for Injection (BWFI) in the multiple use 440 mg vial
containing the lyophilised trastuzumab, directing the stream
into the lyophilised cake.
-
Swirl the vial gently to aid reconstitution. DO NOT SHAKE!
Slight foaming of the product upon reconstitution is not
unusual. Allow the vial to stand undisturbed for approximately 5
minutes. The reconstituted trastuzumab results in a colourless
to pale yellow transparent solution and should be essentially
free of visible particulates.
Multiple dose vial (Multi-use vial) 150 mg
Each 150 mg vial of trastuzumab is reconstituted with 7.2 mL of
Bacteriostatic Water for Injection (BWFI), USP, containing 1.1%
benzyl alcohol as a preservative to yield a multi-dose solution
containing 21 mg/mL trastuzumab. In patients with known
hypersensitivity to benzyl alcohol, reconstitute with 7.2 mL of
Sterile Water for Injection (SWFI) without preservative to yield
a single use solution.
Use of other reconstitution solvents should be avoided.
Trastuzumab should be carefully handled during reconstitution.
Causing excessive foaming during reconstitution or shaking the
reconstituted solution may result in problems with the amount of
trastuzumab that can be withdrawn from the vial.
The reconstituted solution should not be frozen.
Instructions for reconstitution:
-
Using a sterile syringe, slowly inject 7.2 mL of
Bacteriostatic Water for Injection (BWFI) in the multiple use
150 mg vial containing the lyophilised trastuzumab, directing
the stream into the lyophilised cake.
-
Swirl the vial gently to aid reconstitution. DO NOT SHAKE!
Slight foaming of the product upon reconstitution is not
unusual. Allow the vial to stand undisturbed for approximately 5
minutes. The reconstituted trastuzumab results in a colourless
to pale yellow transparent solution and should be essentially
free of visible particulates.
Determine the volume of the solution required
-
based on a loading dose of 4 mg trastuzumab/kg body weight, or
a subsequent weekly dose of 2 mg trastuzumab/kg body weight:
Volume (mL) =
Body weight (kg) x dose (4 mg/kg
for loading or 2 mg/kg for maintenance)
21 (mg/mL, concentration of reconstituted
solution)
-
based on a loading dose of 8 mg trastuzumab/kg body weight, or
a subsequent 3-weekly dose of 6 mg trastuzumab/kg body weight:
Volume (mL) =
Body weight (kg) x dose (8 mg/kg
for loading or 6 mg/kg for maintenance)
21 (mg/mL, concentration of reconstituted
solution)
The appropriate amount of solution should be withdrawn from the
vial and added to an infusion bag containing 250 mL of 0.9 %
Sodium chloride solution. Do not use with glucose-containing
solutions. The bag should be gently inverted to mix the solution
in order to avoid foaming. Once the infusion is prepared, it
should be administered immediately. If diluted aseptically, it
may be stored for 24 hours (do not store above 30°C).
Parenteral medicinal products should be inspected visually for
particulate matter and discoloration prior to administration.
Any unused medicinal product or waste material should be
disposed of in accordance with local requirements.
No incompatibilities between trastuzumab and polyvinylchloride,
polyethylene or polypropylene bags have been observed.
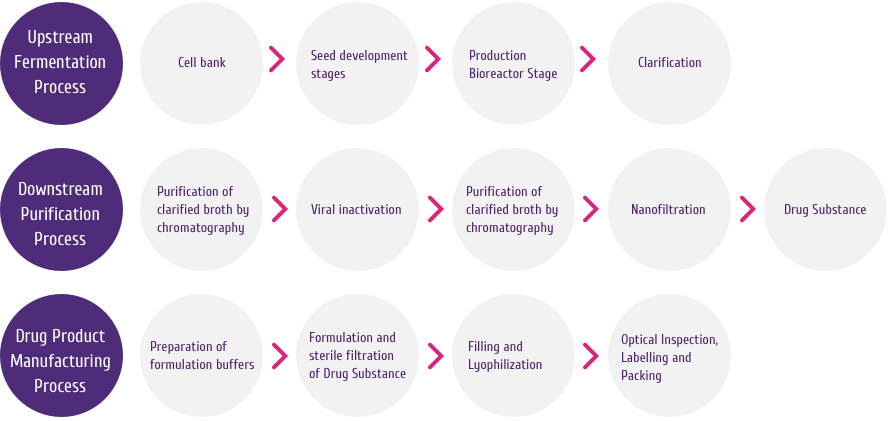
Packaging Benefits
HERVYCTA® is available as a pluspack comprising
one vial of Trastuzumab lyophilized powder and one vial of
bacteriostatic water for injection (BWFI). The drug vial, the
diluent vial and the pack offer some unique benefits that allow
safe and convenient use of HERVYCTA®.
HERVYCTA® (trastuzumab) is available in multiple
dose vials (150 mg/ml and 440 mg/vial).
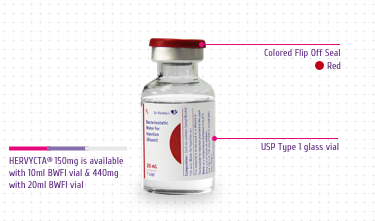
Drug Vial
The drug vial for Hervycta® has following
features offering advantages to the physicians and patients:
-
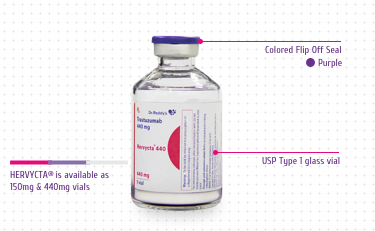
Colored Flip Off Seal: For differentiation
among drug vial and diluent vial
-
USP Type 1 Glass Vial: For product integrity
Bacteriostatic water for injection Vial
Each presentation of Hervycta® contains the drug
vial and a BWFI vial. This facilitates easy reconstitution of
the lyophilized drug and stability of reconstituted drug for a
period of 28 days post reconstitution.
The diluent vial has following features offering advantages to
the physicians and patients:
-
Colored Flip Off Seal: For differentiation
among drug vial and diluent vial
-
USP Type 1 Glass Vial: For product integrity
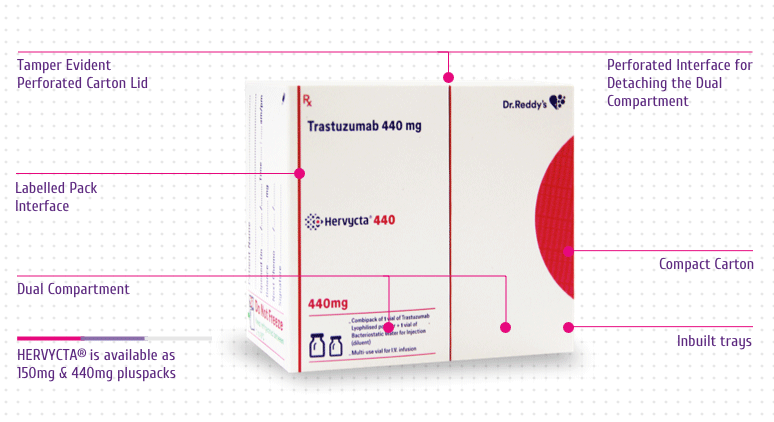
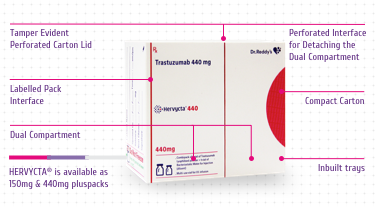
Pluspack
Hervycta® vials are supplied in a unique
differentiated pack to ensure product integrity and convenience
for physicians, nurses and patients.
The unique pack designed for Hervycta®
encompasses multiple novel features with specific advantages as
mentioned below (Figure 7):
-
Compact Carton - For optimum space
utilization while storing the drug (before or after
reconstitution)
-
Dual Compartment - Drug and diluent vials are
stored in separate chamber which safeguards each vial and
avoids breakage
-
Inbuilt Tray – For safe holding of the two
vials to avoid any movement or breakage
-
Tamper Evident Perforated Carton Lid – A
visible tamper evidence at carton opening
-
Perforated Interface for Detaching the Dual Compartments
–
Easy compartment tear off for retention and storage of unused
reconstituted drug solution with ample protection and better
space utilization
-
Labelled Pack Interface – Useful for
capturing information related to the drug (unused or
reconstituted drug) along with patient details
-
Micro Embossing and Micro Text - Anti
counterfeit features
Abbreviations
- BWFR : Bacteriostatic water for reconstitution
- EBC : Early breast cancer
- HER2 : Human epidermal growth factor 2
- HER2+ : Human epidermal growth factor 2 positive
- IgG1 : Immunoglobulin sub-class G1
- LVEF : Left ventricular ejection fraction
- MBC : Metastatic breast cancer
- MGC : Metastatic gastric cancer
References
-
Carter P, Presta L, Gorman CM, et al. Humanization of an
anti-p185HER2 antibody for human cancer therapy. Proceedings of
the National Academy of Sciences of the United States of
America. 1992;89(10):4285-4289.
-
Schechter AL, Stern DF, Vaidyanathan L, et al. The neu oncogene:
an erb-B-related gene encoding a 185,000-Mr tumour antigen.
Nature 1984;312:513–6
-
Yarden Y, Biology of HER2 and Its Importance in Breast Cancer.
Oncology 2001;61(suppl 2):1-13
-
Sylvie Menard, Serenella Maria Pupa, Manuela Campiglio, Elda
Tagliabue, Biologic and therapeutic role of HER2 in cancer.
Oncogene (2003) 22, 6570. doi:10.1038/sj.onc.1206779
-
Hudis CA, Trastuzumab – mechanism of action and use in clinical
practice, N Engl J Med., 2007, Jul 5:357(1):39-51
-
HERVYCTA® (Trastuzumab) Prescribing information,
Dr. Reddy’s.